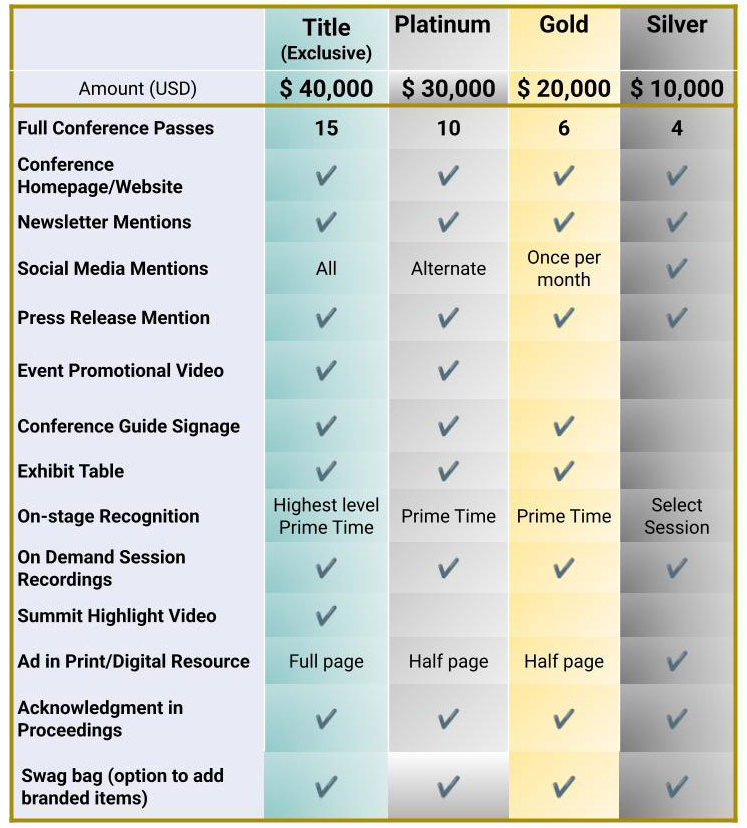
Sponsorship Matrix
Sponsorship A La Carte

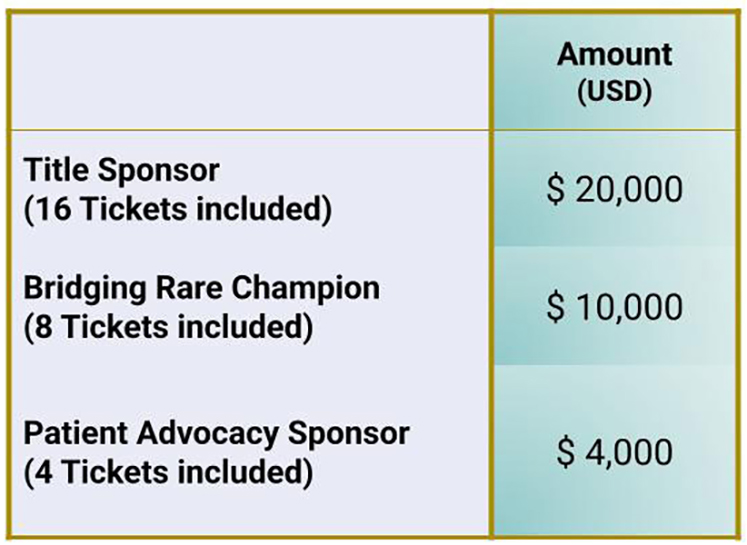
Gala Sponsorship
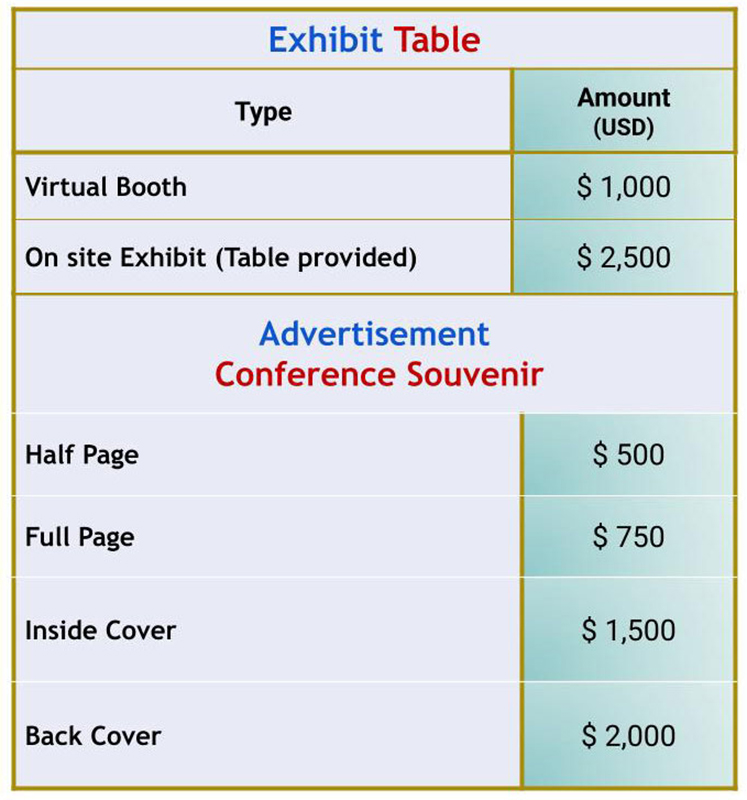
Exhibits & Advertisements




Frank J. Sasinowski, has played a pivotal role in securing FDA approval for hundreds of new drugs, including over 100 new molecular entities, many of which target serious and rare diseases. Notably, he has contributed to over half of all non-oncology drugs approved through the FDA's accelerated approval pathway and has been instrumental in numerous cell and gene therapies, including the first systemic gene therapy, Zolgensma, and Lenmeldy. His expertise extends to championing the alternative effectiveness statutory standard, as seen in approvals of therapies like Duvyzat, Miplyffa, Skyclarys, Filsuvez, and Vyjuvek. Frank began his career at the FDA in 1983 as regulatory counsel in the Center for Drugs and Biologics, where he contributed to the implementation of the 1983 Orphan Drug Act and the 1984 Hatch-Waxman Act.
His influential work includes a seminal 2012 Drug Information Journal analysis titled “Quantum of Effectiveness Evidence in FDA’s Approval of Orphan Drugs: Cataloguing FDA’s Flexibility in Regulating Therapies for Persons with Rare Disorders,” which continues to be widely cited across the FDA, industry, and academia. Frank’s extensive knowledge of FDA processes, coupled with his advocacy for patient-centered drug development, grants him a unique perspective on regulatory matters. Since 2014, he has served as an Adjunct Professor of Neurology at the University of Rochester School of Medicine and has contributed his insights to numerous boards, including the National Organization for Rare Disorders (NORD) and the EveryLife Foundation for Rare Diseases. His achievements have been recognized with numerous awards, including NORD’s inaugural Lifetime Achievement Award, the FDA Award of Merit, and the 2021 Pope Francis Pontifical Hero Award for Inspiration.
Frank's academic journey includes a B.S. in Biological Sciences and Genetics from Cornell University, an M.S. in Nutritional Sciences, and an M.P.H. from the University of California at Berkeley, followed by a J.D. from Georgetown University Law Center. His influence and dedication are also reflected through his work with the IndoUSrare patient organization and on the boards of the United States Pharmacopeia (USP) and the ARM Foundation.
Dr. Gahl’s extensive research portfolio has been dedicated to rare inborn errors of metabolism. His research approach encompasses both clinical observations and treatment of patients in the clinic and intricate biochemical, molecular biological, and cell biological investigations in the laboratory.
His groundbreaking work has led to the elucidation of the fundamental defects in rare genetic disorders, including cystinosis and Salla disease. Notably, these discoveries have contributed to the development of innovative therapies, such as cysteamine, which gained approval by FDA for the treatment of cystinosis, improving the lives of patients affected by this rare condition.
Dr. Gahl’s commitment to advancing medical knowledge is evident in his extensive publication record, which includes 450+ peer-reviewed papers. His mentorship has been equally impactful, as he has trained 42 biochemical geneticists, nurturing the next generation of experts in the field. Moreover, he extends to leadership roles within prominent medical organizations. He has served on the board of directors of the American Board of Medical Genetics and Genomics and the American Society of Human Genetics. His presidency of the Society for Inherited Metabolic Disorders further underscores his leadership and advocacy efforts within the rare disease community.
He has served on the board of directors of the American Board of Medical Genetics and Genomics and the American Society of Human Genetics. His presidency of the Society for Inherited Metabolic Disorders further underscores his leadership and advocacy efforts within the rare disease community.
Dr. Gahl has been the recipient of Dr. Nathan Davis Award for Outstanding Government Service: Presented by the American Medical Association (AMA), Service to America Medal in Science and the Environment and Election to the National Academy of Medicine. In addition to these accolades, Dr. Gahl received the EURORDIS Lifetime Achievement Award, a prestigious acknowledgment of his lifelong dedication to addressing the needs of individuals living with rare and undiagnosed diseases. This award recognizes his pivotal role in the creation of the National Institutes of Health (NIH) Undiagnosed Diseases Network (UDN) and his leadership in the development of the International Network on Undiagnosed Diseases (UDNI). These initiatives have significantly impacted rare disease research on a global scale.
Dr. Ishwar Chander Verma, shortly known as IC Verma, is currently Advisor and Senior Consultant at the Institute of Medical Genetics and Genomics at Sir Ganga Ram Hospital in Delhi. He has spent a lifetime working on raredisorders; from establishing their burden to their diagnosis and prevention. For his stellar contributions in this field, he received the BC Roy – Medical Council of India National Award. He chaired the committee that prepared the first draft of the National Policy for Rare Disorders in India.
He set up the first Genetic center in India – at AIIMS, Delhi in 1968, the first registry for genetic disorders, and was the first to provide advanced genetic tests at low cost using indigenous methods.
Dr. Verma obtained medical education at Amritsar Medical College, post graduate training in medicine and child health from the Royal College London, and Glasgow University. He received Genetic training in USA at MGH in Boston and NIH. He has been involved in a number of Indo-US projects on rare disorders – Genetic studies in deafness with NIH, and Birth defects with March of Dimes and CDC, Atlanta.
He served as Professor of Pediatrics at AllMS, New Delhi which is a premier Medical institute in India. The Genetic unit he established was recognized as WHO Collaborating Center in Genetics, the only one in Asia. After retirement developed a state-of-theart- genetic center in Sir Ganga Ram Hospital in 1997. He has been involved for 20 years in investigating rare disorders among tribal communities in Andaman and Nicobar Islands and other regions in India. He has provided prenatal diagnosis and counseling for the prevention of genetic disease to thousands of patients with rare disorders.
He was instrumental in starting a number of patient societies - for thalassemia, mental retardation, muscular dystrophy etc. Dr. Verma has 457 research publications to his credit. He has been editor-in-chief of the Indian Journal of Pediatrics for 25 years, a journal that has the highest Impact Factor of 4.2 among all medical journals in India.
He has fellowship of National Academy of Medical Sciences, India, Fellowship of American Academy of Pediatrics and American Pediatric Research Society. He has served as president of Indian Society of Human Genetics, Indian Academy of Pediatrics Delhi, Society of Fetal Medicine and Indian Society of IEMs. He has made immense contributions to improve the health of patients with genetic diseases and rare disorders in India, for which he has been conferred Padmashri by the President of India in 2023.
Dr. Hasmukh Jain is a hemato-oncologist, currently working as a professor at Tata memorial centre, Mumbai. He completed his MD medicine from KMC Mangalore and DM medical oncology from the prestigious Tata memorial centre. His research work is focussed on ALL, Hodgkin’s lymphoma, artificial intelligence and cell therapy. His research has been published in national and international journals.
He was the lead PI on the pivotal trials that led to the approval of CAR-T cell therapy in India and is conducting pioneering research to study CAR-T cell therapy in the frontline and improve access.
He has received several awards in his career including the best post graduate student in internal medicine from manipal university, awards on CAR-T cell therapy research from haematology cancer consortium, indian society of medical and paediatric oncology and European group for blood and marrow transplantation.
Dr. Rahul Purwar is a Professor at the Department of Biosciences and Bioengineering at IIT Bombay. He holds a PhD in Molecular Medicine from Hannover Medical School, Germany. Dr. Rahul’s professional journey spans various prestigious institutions and roles. He started his post-doctoral fellowship at Harvard Medical School, Boston, USA. After his fellowship, he joined ImmunoGen, Inc. (Waltham, MA, USA), as a scientist before returning to India as a faculty member at IIT Bombay.
Based on his research at IIT Bombay, Dr Purwar founded ImmunoACT in 2018, first cell & gene therapy company in India. In this role, he drives strategic initiatives through accelerated process & clinical development and manufacturing, which has not only led to the successful market authorization of the country’s first CAR-T cell therapy, but also one that is fully Indigenous to India. This is the first of many planned milestones in democratizing access to advanced cell & gene therapies in India, his fundamental vision and founding principle of ImmunoACT.
Dr. Purwar’s research work is well-recognized in the scientific community, with numerous publications to his credit in esteemed journals. His contributions to the field of Biosciences & Bioengineering are commendable and his work continues to inspire many in the scientific community.